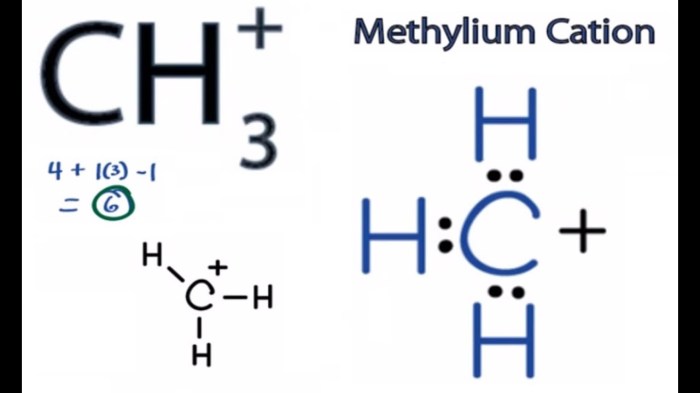
Rank the alkenes below from most stable to least stable embarks on a journey of discovery into the realm of alkene stability. This discourse delves into the intricacies of alkene structure, delving into the factors that govern their relative stabilities, promising an adventure of scientific exploration and enlightenment.
Alkene stability, a cornerstone of organic chemistry, hinges on a delicate interplay of electronic and structural attributes. This discourse will illuminate the influence of alkyl groups, electronegative substituents, branching patterns, and ring strain on alkene stability, unraveling the complexities that shape their chemical behavior.
Overview of Alkene Stability
Alkenes are hydrocarbons that contain at least one carbon-carbon double bond. They are generally more stable than alkanes, which have only single bonds between carbon atoms. The stability of an alkene depends on several factors, including the number and type of substituents on the double bond and the overall structure of the molecule.
Substituent Effects on Alkene Stability, Rank the alkenes below from most stable to least stable
The presence of alkyl groups on an alkene double bond increases the stability of the alkene. This is because alkyl groups are electron-donating groups, which means they can donate electrons to the double bond and help to stabilize it. The more alkyl groups that are attached to the double bond, the more stable the alkene will be.
Electronegative groups, such as halogens and oxygen, have the opposite effect on alkene stability. These groups can withdraw electrons from the double bond, which makes the alkene less stable. The more electronegative groups that are attached to the double bond, the less stable the alkene will be.
Structural Effects on Alkene Stability
The structure of an alkene can also affect its stability. Branched alkenes are less stable than unbranched alkenes. This is because the branched structure creates more steric hindrance around the double bond, which makes it more difficult for the alkene to react.
Ring strain can also affect the stability of an alkene. Alkenes that are part of a ring are less stable than alkenes that are not part of a ring. This is because the ring strain in the alkene makes it more difficult for the alkene to react.
Common Queries: Rank The Alkenes Below From Most Stable To Least Stable
What is the most stable alkene?
The most stable alkene is the one with the most substituted double bond, as this reduces the number of reactive hydrogens and increases the number of electron-donating alkyl groups.
What is the least stable alkene?
The least stable alkene is the one with the least substituted double bond, as this increases the number of reactive hydrogens and decreases the number of electron-donating alkyl groups.
How do you rank alkenes from most stable to least stable?
To rank alkenes from most stable to least stable, consider the following factors: the number of alkyl groups attached to the double bond, the electronegativity of any substituents on the double bond, the branching pattern of the alkene, and the presence of any ring strain.