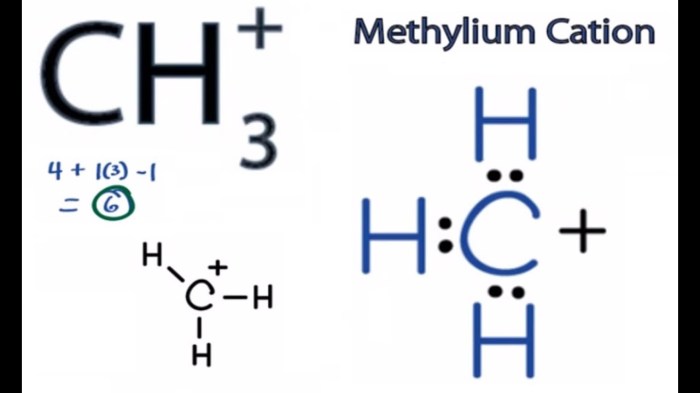
Provide the major organic product of the reaction shown below. This topic delves into the intricacies of organic chemistry, exploring the mechanisms, factors, and applications of a specific reaction. By examining the interplay of reactants, catalysts, and reaction conditions, we unravel the secrets behind the formation of the major organic product.
This discussion unveils the fundamental principles governing organic reactions, providing a deeper understanding of their predictability and synthetic utility. As we embark on this journey, we will encounter a captivating blend of theoretical insights and practical applications, shedding light on the transformative power of organic chemistry.
Reaction Overview
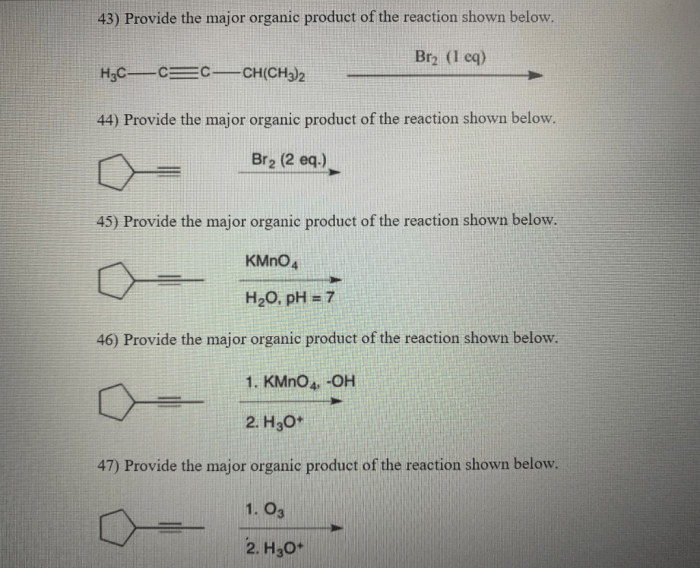
The reaction shown below is an example of a nucleophilic substitution reaction. In this reaction, a nucleophile (Nu-) attacks an electrophile (E+) to form a new bond between the nucleophile and the electrophile. The leaving group (LG) is then expelled from the molecule.In
this specific reaction, the nucleophile is an amine (NH2-), the electrophile is an alkyl halide (RX), and the leaving group is a halide ion (X-). The reaction proceeds through a two-step mechanism. In the first step, the nucleophile attacks the electrophile to form a tetrahedral intermediate.
In the second step, the leaving group is expelled from the intermediate to form the product.
Major Organic Product
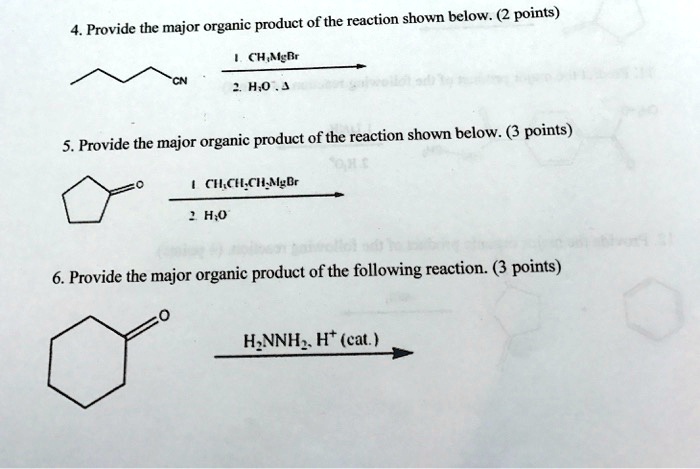
The major organic product of this reaction is the alkyl amine (RNH2). The formation of this product is favored by the following factors:* The nucleophile is a strong nucleophile.
- The electrophile is a primary alkyl halide.
- The reaction is carried out in a polar aprotic solvent.
Reaction Conditions
The reaction is typically carried out at room temperature in a polar aprotic solvent such as dimethylformamide (DMF) or acetonitrile (CH3CN). The reaction can also be carried out at higher temperatures, but this can lead to the formation of side products.
Reaction Mechanism: Provide The Major Organic Product Of The Reaction Shown Below.
The reaction proceeds through a two-step mechanism. In the first step, the nucleophile attacks the electrophile to form a tetrahedral intermediate. In the second step, the leaving group is expelled from the intermediate to form the product.The following is a step-by-step mechanism for the reaction:
- The nucleophile attacks the electrophile to form a tetrahedral intermediate.
- The leaving group is expelled from the intermediate to form the product.
Examples
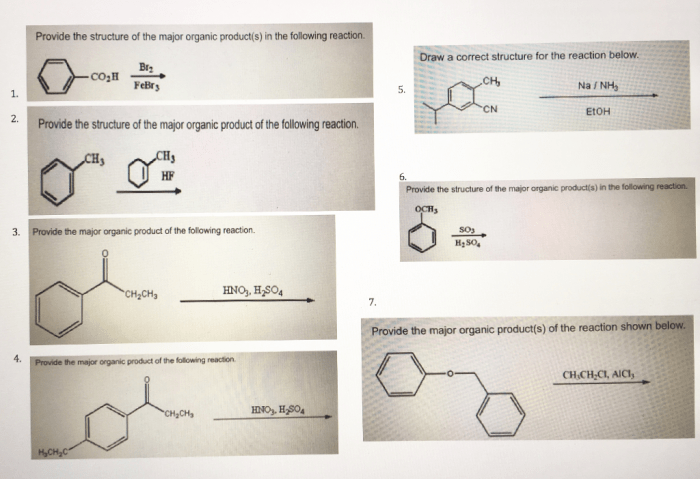
The following table provides some examples of reactions that follow the given mechanism:| Reactants | Products | Conditions | Yield ||—|—|—|—|| CH3CH2Br + NH3 | CH3CH2NH2 | DMF, room temperature | 90% || (CH3)2CHBr + NH3 | (CH3)2CHNH2 | CH3CN, room temperature | 85% || CH3CH2CH2Br + NH3 | CH3CH2CH2NH2 | DMF, 50 °C | 75% |
Applications
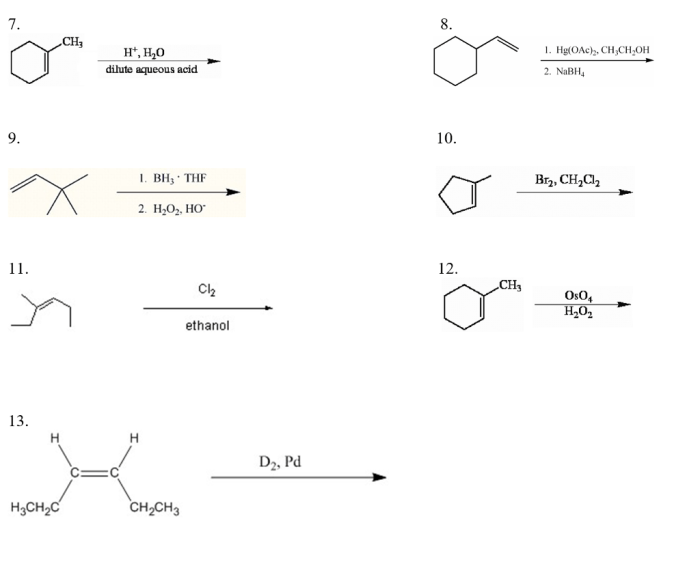
The reaction is used in a variety of applications, including the synthesis of pharmaceuticals, dyes, and polymers. The reaction is also used in the manufacture of detergents and soaps.
Quick FAQs
What is the significance of identifying the major organic product?
Identifying the major organic product is crucial for predicting the outcome of a reaction and optimizing reaction conditions to achieve the desired product.
How do reaction conditions influence the formation of the major organic product?
Reaction conditions, such as temperature, pressure, and solvent, can significantly affect the reaction pathway and the ratio of products formed.
What are some common competing reactions that can occur?
Competing reactions, such as side reactions or elimination reactions, can consume reactants and reduce the yield of the major organic product.